When completed, the system will test for the SARS-CoV-2 virus in as few as 10 minutes and can be performed in healthcare facilities or the workplace.


As new cases of COVID-19 surge across the country, states have ramped up their testing efforts, but increased testing and overburdened laboratories often means patients have to wait a week or more to find out if they’re positive.
A team of Hopkins engineers is developing a solution to this problem: a biosensing platform that not only can test for viruses—including SARS-CoV-2, the virus that causes COVID-19—in as few as 10 minutes, but also does not require expensive, specialized laboratory equipment or expertise. Experts agree that effectively managing the current pandemic—and future infectious disease outbreaks—will require point-of-care testing that can detect virus strains in mere minutes.
“We envision a cost-effective testing method that can be performed onsite by healthcare workers in a clinic or in the workplace. That could be a huge advantage for scaling up population-wide testing, especially in low-resource areas,” said David Gracias, a professor of chemical and biomolecular engineering at the Whiting School of Engineering. Gracias has partnered with Ishan Barman, an associate professor of mechanical engineering, on the project.
Identifying specific viruses in human specimens is challenging. Furthermore, viruses can evolve, and there’s growing evidence that SARS-CoV-2 is doing just that, says Barman, a molecular spectroscopy expert.
By combining expertise in nanofabrication, optical spectroscopy, biosensing, and machine learning, the Hopkins team plans to create a next-generation diagnostic tool capable of keeping pace with emerging and evolving viruses.
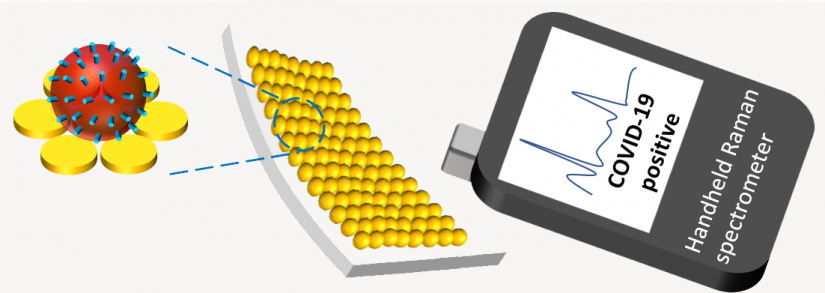

Image caption:The team’s novel testing platform, which uses Surface-enhanced Raman spectroscopy on nanomanufactured material, will be able to rapidly detect the presence of a virus, even if only small traces exist in the sample
IMAGE CREDIT: KAM SANG KWOK, JOHNS HOPKINS UNIVERSITY
To do this, Gracias, along with graduate student Kam Sang Kwok, is using state-of-the-art nanomanufacturing techniques to design and fabricate large-area, flexible sensor material. Using nanoimprint lithography—a method of fabricating nanometer scale patterns—and strain engineering to alter the final dimensions of the patterns, they will construct virus-trapping, nano-sized gaps with a dimension of less than 10 nm on the material. When a nasal or saliva sample is placed on this material, these nanogap substrates act as tiny “lightning rods” that enhance the electromagnetic field near the virus particles.
Next, Barman and postdoc Debadrita Paria will analyze the sample using Surface-enhanced Raman spectroscopy, which employs laser light to examine how molecules vibrate. Because the nanostructured material strengthens the virus’s Raman signal significantly, the team believes the system will be able to rapidly detect the presence of a virus, even if only small traces exist in the sample.
According to Barman, the system’s key innovation is the incorporation of a machine learning model that detects very subtle features in the data. Training the model to identify and distinguish COVID-19 from other respiratory illnesses will reduce false negative outcomes, another weakness of current testing methods.
“Viruses strike suddenly, and that’s why we are where we are today with COVID-19. For future waves of the outbreak, it will be crucial to have near real-time testing available,” said Barman. “Label-free optical detection, combined with machine learning, allows us to have one platform that can test for a wide range of viruses with enhanced sensitivity and selectivity.”
Gracias and Barman say their technology could someday be integrated into a hand-held testing device, much like the temperature guns now ubiquitous in clinics and stores.
The work is in its early stages, and the team hopes to have a proof of concept ready in the next several months. With sufficient sensitivity and selectivity, their method represents a significant advance in the detection and monitoring of new pathogenic viruses.
“It’s early in the game, but we’re excited because our idea is grounded in solid science” said Gracias. “We’re building on things we already do in our labs, and we’re synergizing our skills to uncover a new approach to a fundamental problem.”
The project is being developed with the support of a $200,000 National Science Foundation EAGER grant, under a program supporting exploratory, early-stage research on untested, but potentially transformative, ideas or approaches.



































