A research team led by William Chao Chong Hang, associate professor in the Faculty of Health Sciences (FHS), University of Macau (UM), has identified a new type of V. parahaemolyticus toxin RhsP, which uncovers new insights into Rhs-type toxin release triggered by autoproteolysis and has laid the foundation for understanding how V. parahaemolyticus infects human guts by killing and outcompeting the host microbiota and causes gastroenteritis. The research results have been published in the internationally renowned journal Cell Reports.

About 2.4 billion people worldwide contract gastroenteritis each year and the major cause of the disease is the consumption of seafood contaminated with the Gram-negative bacterium Vibrio parahaemolyticus. In order to gain entry to and colonise the human guts, V. parahaemolyticus releases toxins through its type-VI secretion system (T6SS) to kill the gut microbiota, thereby creating a favourable environment for itself in the human guts. The research team led by Prof Chao’s research team identified a new type of V. parahaemolyticus toxin RhsP, which releases a C-terminal nuclease domain RhsPC to kill neighbouring bacteria. The bacterium protects itself from its own RhsP toxin by producing a cognate immunity protein RhsPI to avoid suicide. The RhsP toxin is a huge protein with 1381 amino acids and a molecular weight of 157kDa. It is mainly composed of a huge β barrel structure, which encapsulates key catalytic elements required for autoproteolysis.
Supported by the Shanghai Synchrotron Radiation Facility of the Chinese Academy of Sciences, the research team applied X-ray crystallography to determine the atomic structure of the RhsPC-RhsPI toxic-immunity pair, illustrating how the immunity protein RhsPI protects the host by utilising its acidic surface to block the basic nuclease active site. This is the first protein complex structure determined by X-ray crystallography in Macao.


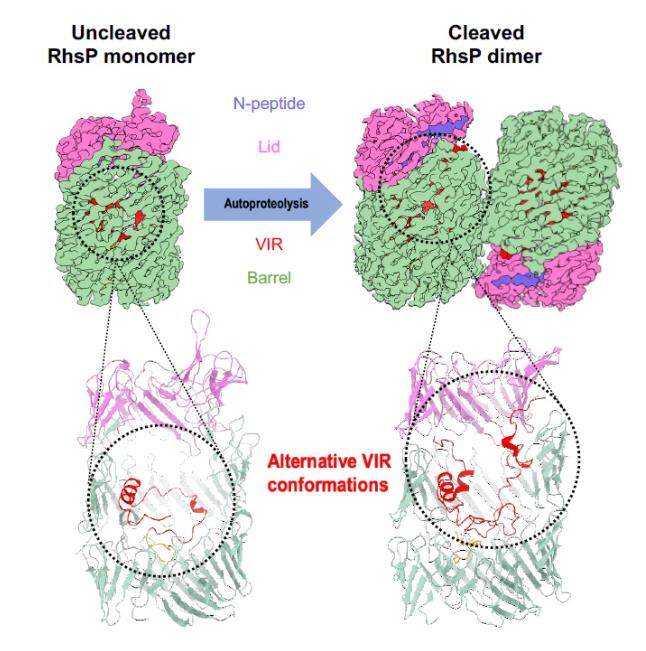
In addition, the research team collaborated with a team led by Dr He Jun, a researcher at the Guangzhou Institute of Biomedicine and Health (GIBH) of the Chinese Academy of Sciences, to apply cutting-edge cryo-electron microscopy (cryo-EM) techniques to determine the structures of the RhsP toxin in its pre-autoproteolysis and post-autoproteolysis forms. The team discovered that autoproteolysis triggers a dramatic RhsP conformational change, which promotes its own dimerization. Using complementary genetic experiments, the team confirmed that RhsP dimerization is required for the release of the C-terminal toxic nuclease and the T6SS2-mediated prey targeting by V. parahaemolyticus. These structures are the first cryo-EM protein structures determined in Macao.
The corresponding authors of this study are Prof Chao, Dr He, and Zheng Jun, former associate professor in the FHS. The first authors include former UM postdoctoral fellows Tang Le and Nadia Rasheed, UM PhD student Wu Hao Weng, GIBH PhD student Dong Shuqi, and research assistant Zhou Ningkun. The project was funded by the Science and Technology Development Fund of the Macao SAR (File No: 0009/2018/A1, 0058/2018/A2, 0113/2019/A2 and 0032/2021/A1) and UM (File No.: MYRG2018-00221-FHS and MYRG2019-00050-FHS). The full version of the paper can be viewed at https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01610-2.
Source: Faculty of Health Sciences, University of Macau



































